Brand Name
Ztalmy
Generic Name
Ganaxolone
View Brand Information FDA approval date: June 06, 2022
Classification: Neuroactive Steroid Gamma-Aminobutyric Acid A Receptor Positive Modulator
Form: Suspension
What is Ztalmy (Ganaxolone)?
ZTALMY is indicated for the treatment of seizures associated with cyclin-dependent kinase-like 5 deficiency disorder in patients 2 years of age and older. ZTALMY is a neuroactive steroid gamma-aminobutyric acid A receptor positive modulator indicated for the treatment of seizures associated with cyclin-dependent kinase-like 5 deficiency disorder in patients 2 years of age and older.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
ZTALMY (ganaxolone)
1INDICATIONS AND USAGE
ZTALMY is indicated for the treatment of seizures associated with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD) in patients 2 years of age and older.
2DOSAGE FORMS AND STRENGTHS
Oral suspension: 50 mg/mL ganaxolone. Each bottle contains 110 mL of white to off-white cherry flavored suspension.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following important adverse reactions are described elsewhere in the labeling:
- Somnolence and Sedation
- Suicidal Behavior and Ideation
- Withdrawal of Antiepileptic Drugs
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled and uncontrolled trials in patients with seizures associated with CDD, 102 patients were treated with ZTALMY, including 83 patients treated for more than 6 months, and 50 patients treated for more than 1 year.
In Study 1, 50 patients received ZTALMY
The most common adverse reactions (an incidence of at least 5% and at least twice the rate of placebo) were somnolence, pyrexia, salivary hypersecretion, and seasonal allergy (Table 6).
The adverse reactions leading to treatment discontinuation in ZTALMY-treated patients were somnolence and seizure (1 patient) and seizure (1 patient).
Twenty-two percent of ZTALMY-treated patients had dosing interrupted or reduced because of any adverse reaction, compared to 16% of placebo-treated patients. The most frequent adverse reactions leading to a dose interruption or reduction in ZTALMY-treated patients were somnolence (10%) and sedation (2%).
Table 6 presents the adverse reactions that occurred in ZTALMY-treated patients with seizures associated with CDD at a rate of at least 3% and at a rate greater than in placebo-treated patients during the double-blind phase.
5OVERDOSAGE
There is limited clinical trial experience regarding overdose with ZTALMY. Unintentional overdose has been reported in 1 pediatric patient. This patient received ten times the prescribed dose. The patient was hospitalized for evaluation, including an electrocardiogram (ECG) and blood tests, and recovered.
Patients who overdose should be closely monitored and receive standard supportive care. No specific information is available regarding treatment of overdose. In the event of overdose, a certified poison control center should be contacted for updated information on the management of overdose with ZTALMY.
6DESCRIPTION
ZTALMY (ganaxolone) oral suspension contains ganaxolone, a neuroactive steroid gamma-aminobutyric acid A (GABA
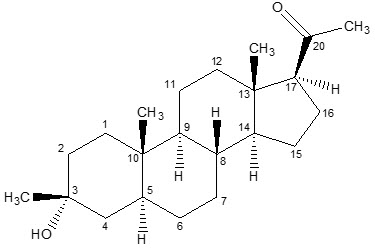
Ganaxolone is a white to off-white crystalline powder that only exists in one crystal form and has low aqueous solubility.
ZTALMY is an oral suspension of ganaxolone. Each mL of oral suspension contains 50 mg of ganaxolone. Inactive ingredients include artificial cherry flavor, citric acid, hypromellose, methylparaben, polyvinyl alcohol, propylparaben, purified water, simethicone emulsion, sodium benzoate, sodium citrate, sodium lauryl sulfate, and sucralose.
7CLINICAL STUDIES
The effectiveness of ZTALMY for the treatment of seizures associated with CDD in patients 2 years of age and older was established in a single, double-blind, randomized, placebo-controlled study in patients 2 to 19 years of age (Study 1, NCT03572933).
Patients enrolled in Study 1 (N=50 for ZTALMY; N=51 for placebo) had molecular confirmation of a pathogenic or likely pathogenic mutation in the CDKL5 gene, seizures inadequately controlled by at least 2 previous treatment regimens, and a minimum of 16 major motor seizures (i.e., bilateral tonic, generalized tonic-clonic, bilateral clonic, atonic, focal to bilateral tonic-clonic) per 28 days during a retrospective 2-month period prior to screening.
Patients were randomized in a 1:1 ratio to receive either ZTALMY or placebo. Following a titration period, patients in the ZTALMY arm weighing 28 kg or less received a maintenance dosage of 21 mg/kg three times daily (with a maximum daily dose of 1800 mg) while patients in the ZTALMY arm weighing more than 28 kg received a maintenance dosage of 600 mg three times daily
Ninety-six percent of patients were taking between 1 to 4 concomitant AEDs. The most frequently used concomitant AEDs (in at least 20% of patients) were valproate (34%), levetiracetam (26%), clobazam (25%), and vigabatrin (22%).
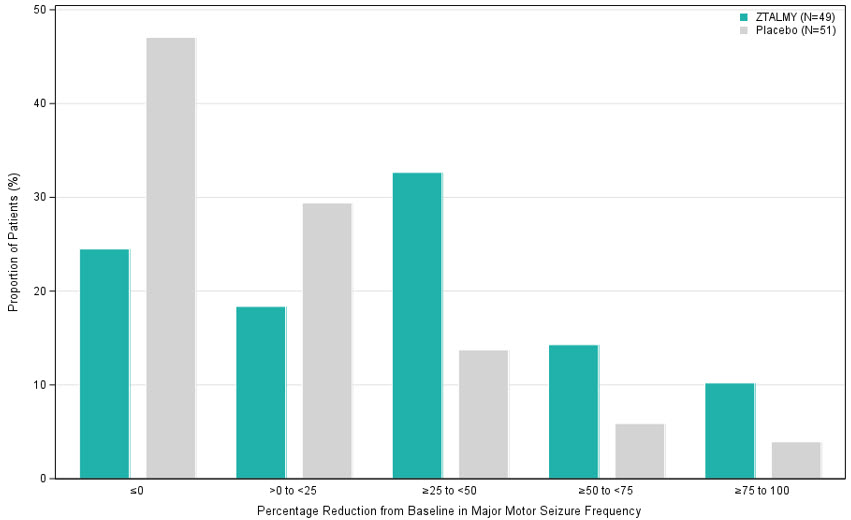
The primary efficacy endpoint was the percentage change in the 28-day frequency of major motor seizures (defined similarly as in the 2-month period prior to screening) from a 6-week prospective baseline phase during the 17-week double-blind phase. Patients treated with ZTALMY had a significantly greater reduction in the 28-day frequency of major motor seizures compared to patients receiving placebo (see
Figure 1 displays the percentage of patients by category of reduction from baseline in the 28-day frequency of major motor seizures during the 17-week double-blind phase.
Figure 1 Proportion of Patients by Category of Seizure Response for ZTALMY and Placebo in Patients with CDD (Study 1)
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
9INSTRUCTIONS FOR USE
ZTALMY ( zuh-tal' mee)
(ganaxolone)
(ganaxolone)
Be sure that you read, understand, and follow these instructions carefully to ensure proper dosing of the oral suspension.
Important:
- Follow your healthcare provider's instructions for how to take or give ZTALMY.
- ZTALMY should always be given with food.
- Ask your healthcare provider or pharmacist if you are not sure how to prepare, take, or give the prescribed dose of ZTALMY.
- Always use the oral syringe provided by your pharmacist to make sure you measure the right amount of ZTALMY.
- Do not use ZTALMY after the expiration date on the package and each bottle.
- Use ZTALMY within 30 days of first opening the bottle.
- After 30 days of first opening the bottle, safely throw away (dispose of) any ZTALMY that has not been used.
Each package contains:
Supplies
- press-in bottle adapter
- oral syringe
You can get a press-in bottle adapter and oral syringes from your pharmacy. Your pharmacist can help you choose the right press-in bottle adapter and oral syringe to use with ZTALMY.
Call your pharmacist right away if you do not have a press-in bottle adapter and the right sized oral syringe to use with your medicine.
Note:If you lose or damage an oral syringe, or cannot read the markings, contact your pharmacist for a new oral syringe.
Follow the instructions below to use the press-in bottle adapter and oral syringe to measure and take or give ZTALMY.
Prepare the bottle
Prepare the dose
Your healthcare provider will tell you how much ZTALMY to take or give.
Take or give ZTALMY
How should I store ZTALMY?
- Store ZTALMY at 68°F to 77°F (20°C to 25°C).
- Always store ZTALMY in its original bottle in an upright position.
- Keep the child-resistant cap tightly closed.
- Use within 30 days of first opening the bottle. Throw away (dispose of) any unused medicine after 30 days.
Keep ZTALMY and all medicines out of the reach of children.
Helpline Details
For additional assistance, call the toll-free helpline at 844-IMMEDICA (1-844-627-4687). Hours: Monday - Friday 8:00 am to 6:00 pm EST
Frequently asked Questions:
Manufactured for Immedica Pharma AB, Stockholm, Sweden
Issued: 11/2025
10PRINCIPAL DISPLAY PANEL - 50 mg/mL Bottle Label
NDC 81583-100-01
Ztalmy
CV
For Oral Administration Only
Recommended Dosage:
Date of first opening
11PRINCIPAL DISPLAY PANEL - 50 mg/mL Bottle Carton
Rx Only
NDC 81583-100-01
Ztalmy
50 mg/mL
CV
For Oral Administration Only
Read enclosed instructions before using
110 mL